Why emergency management should be interested in the emergence of antibiotic resistance
Associate Professor Dale Dominey-Howes, Dr Carolyn Michael , Dr Maurizio Labbate
Abstract
Bacterial epidemics and pandemics are biological risks to life every bit as significant as floods, fires, storms and earthquakes. Antibiotics have been a significant tool in the management of epidemics and pandemics (as well as for fighting general infections) since their discovery in the 1930s. Due to the development of antibiotic resistance by bacteria, we are now approaching a post-antibiotic era where our capacity to manage infectious disease, particularly bacterial epidemics and pandemics, is compromised. Despite considerable efforts by global heath organisations, we need new ways of thinking and acting on the global risk of antibiotic resistance. We argue for a rebranding of the issue to one of a disaster risk and suggest the use of the risk management process and expertise of emergency management to present a new way of thinking about this globally significant risk to life.
Article
Introduction
Disasters take lives, cause devastation, impact individuals, families and communities and disrupt our socio-economic systems (Adger, et al. 2005). Infectious disease resulting in epidemics and pandemics represent a disaster risk to life every bit as significant as fire, flood, storm and other common, high profile disasters. Emergency management agencies under the structure of emergency management and response plans, prepare for, and respond to, sudden and slow onset disasters using an ‘all hazards approach’ and the emergency risk management process. For example, in New South Wales, epidemic and pandemic emergency management arrangements fall to the NSW Ministry of Health via the State Emergency Management Plan (EMPLAN).
Before the 1930s, bacterial infections caused significant morbidity and mortality. Historically, bacteria have been responsible for epidemics and pandemics including the Black Death and Tuberculosis. Antibiotics have saved countless lives, revolutionised medical care (by allowing invasive medical procedures otherwise associated with a high risk of bacterial infection Gottlieb & Nimmo 2011), and provided a powerful tool to aid in the fight of bacterial epidemics and pandemics. However, bacteria have become resistant to antibiotics and we are reaching a ‘post-antibiotic era’ where bacterial infections will be difficult, if not impossible to treat (Prasad & Smith 2013, CDC 2013, WHO 2012).
As a slow onset disaster, antibiotic resistant infections are rising and already affect millions of people globally (WHO 2012). Sudden onset antibiotic resistant outbreaks regularly occur in hospitals, affecting the most vulnerable and require costly interventions. In the United States for example, over two million people are affected by antibiotic resistant infections with at least 23 000 deaths annually. This equals US$20 billion in extra healthcare costs and US$35 billion in lost productivity (CDC 2013). Such statistics have led to calls to urgently address this problem coming from, among others, the World Health Organization (WHO) and the Center for Disease Control (CDC) (CDC 2013, WHO 2012) and from respected individuals such as the UK Chief Medical Officer, Professor Dame Sally Davies (Walsh 2013) and the Chief Australian Scientist (Prasad & Smith 2013). They have also become increasingly urgent with Professor Dame Sally Davies calling for antibiotic resistance to be placed on the risk register ahead of terrorism (Walsh 2013).
For sudden onset bacterial epidemics and pandemics, antibiotics are one of the major management tools. The emergence of antibiotic resistance means that the capacity of health systems to manage the associated disaster risk is more complicated. The risk is heightened by poor community awareness of the problem of antibiotic resistance (Francis, et al. 2012).
How the antibiotic resistance problem has developed
Bacteria, among the oldest forms of life, are mostly harmless to humans and are essential for a healthy environment (e.g. nutrient cycling) and body (e.g. production of vitamins). Only a minority are pathogenic and, so, deleterious to humans. Bacteria are remarkably resilient and adaptable and have managed to occupy almost every ecological niche overcoming all manner of environmental challenges. Thus, as foretold by the discoverer of Penicillin, Alexander Fleming, resistance to antibiotics was always a given. Particularly striking is the rate at which bacteria have evolved antibiotic resistance. In just 70 years since the first use of Penicillin, some bacterial infections are no longer treatable (CDC 2013, WHO 2012). In this context, humanity is largely to blame. Much has been said about the reasons for the resistance problem (CDC 2013, WHO 2012) which is highly complex. However, in its most basic form, antibiotic use leads to antibiotic resistance.
Widespread use, overuse and misuse of antibiotics in multiple settings including medicine, agriculture, and animal husbandry to prevent infection (i.e. prophylaxis), or as a method of promoting increased animal growth, has created strong selective pressures in favour of bacteria that resist antibiotics. Consequently, resistant bacteria are found increasingly on and in humans, farm animals, seafood, fruit and vegetables and in the environment (Baquero, Mrtinez & Cantón 2008, Kemper 2008, Wright 2010). Bacteria acquire antibiotic resistance in two ways. The first is by genetic mutation. The second is by actually passing genetic material containing genes that provide resistance between bacteria — a process called horizontal gene transfer (HGT). The consumption of food or direct contact with people or environments containing antibiotic resistant bacteria can cause normal healthy bacteria in and on humans to acquire resistance via HGT. This acquired resistance may then spread through human communities and may be passed on to disease-causing bacteria during an infection. Once resistance has been acquired by a pathogenic bacterium it is maintained by the continued pressure of antibiotic use, as observed by the levels of resistance in hospitals where antibiotics are commonly used. Through antibiotic use, overuse and misuse, we have added unnecessary fuel to the fire of antibiotic resistance and decreased the shelf life of these important drugs.
Antibiotics are among the most commonly prescribed drugs in human medicine and it is estimated that up to 50 per cent of all the antibiotics prescribed are unnecessarily or ineffectively prescribed (CDC 2013). In Australia, 22 million scripts are written for antibiotics each year. It is extremely likely that a significant number are unnecessary due to inappropriate prescription for, among other reasons, viral infections — a distinction not often understood by the public, health practitioners and the media. In fact, a National Prescribing survey found 62 per cent of patients did not know that overusing antibiotics increased resistance (NPSMW 2013). To date, resistance to one antibiotic was circumvented by the development of new antibiotics. However, following the development of each new antibiotic, bacteria have evolved or acquired new resistance capability. Unfortunately, we can no longer rely on the development of new antibiotics because their pharmaceutical development has stalled due to economic and regulatory reasons (Power 2006). Drug discovery and development is expensive and it is estimated that ~US$1 billion is spent before a drug reaches the market (Power 2006). Given the speed at which bacteria become resistant, a pharmaceutical company may only achieve circa five years of revenue before drug effectiveness begins to diminish. Furthermore, courses of treatment are short (typically one week) so the capacity to generate profit is modest. Consequently, society is left with a situation where the current suite of antibiotics is losing its effectiveness and there is a dearth of new antibiotics coming onto the market. Unless something changes quickly, the post-antibiotic era of untreatable bacterial infections, including epidemics and pandemics, is inevitable.
Addressing the antibiotic resistance problem
The problem of antibiotic resistance has not been ignored and expert panels in consultation with governments have made recommendations for reducing the burden of antibiotic resistance. In the most recent WHO report (WHO 2012), recommendations included:
- Preventing bacterial infections and the spread of antibiotic resistance: Implementation of good hygienic practices is effective in controlling the spread of bacterial infections. Regular hand washing can prevent infections being spread. Furthermore, isolation of hospital patients infected with or detected as carrying resistant bacteria is advised.
- Surveillance of antibiotics and antibiotic resistance: Through effective surveillance of antibiotic use and antibiotic resistance, policies and actions may be developed and implemented to control antibiotic use and contain resistance outbreaks. Surveillance of antibiotic resistance can guide prescription in life-and-death medical situations to ensure appropriate selection of an effective antibiotic.
- Antibiotic stewardship: Proper administration of antibiotics by heath practitioners would reduce the selection pressure on bacteria and reduce the emergence of resistance. This includes not using antibiotics when they are not effective and selecting the most appropriate antibiotic for specific infections. Part of the solution is better education of the problem so that appropriate behaviour regarding the use of antibiotics is encouraged.
- Reduce use of antibiotics in agriculture: The use of antibiotics for growth promotion in animals adds unnecessary pressure. As a source of human food, there is direct contact between humans and antibiotics and antibiotic resistant bacteria that facilitates the spread. Even more concerning is that resistant human pathogens are commonly found on food animals. Some countries have banned the use of antibiotics in agriculture but the use is still widely practiced.
- Development of new antibiotics, vaccines and other treatments: More investment from governments into research and development and greater incentive for industry to invest in the development of new antibiotics and vaccines is required.
- Development of improved diagnostic tests of bacterial infections: To assist improved antibiotic stewardship and surveillance, cheaper and more rapid methods to identify specific bacterial infections and detecting antibiotic resistance are required.
Many of these recommendations have been implemented globally. However, their effectiveness is dependent on their enforcement and whether resources are available to implement them. With the declining effectiveness of antibiotics there is a future risk that control of bacterial infectious outbreaks will be troublesome. Already we are seeing hospitals regularly dealing with outbreaks of superbugs requiring ring-fencing of vulnerable patients (barrier nursing), increased cleaning procedures, and increased surveillance of superbugs on patients, hospital staff and the hospital environment. Hospital-acquired (nosocomial) infections, most of which are multi-drug resistant, affect 5-10 per cent of hospital patients costing in excess of AU$2-3 billion (Gilbert, Iredelle & Merlino 2014). At the moment, the vulnerable are at most risk of untreatable nosocomial infections because of compromised immune systems and being exposed to invasive medical techniques such as surgery and catheter use that facilitate direct access of bacteria to the bloodstream.
It seems unlikely that the recommendations previously listed will stem the flow of the emergence of antibiotic resistance. However, it is clear that dealing with the problem is complex and requires a multi-pronged approach that involves consultation and co-operation among stakeholders.
Rebranding antibiotic resistance as a disaster risk management problem
To date, responsibility for responding to the slow onset risk of antibiotic resistance has fallen to the health and medical industries. Globally, health authorities and practitioners have done a tremendous job. But, given the scale of the looming crisis, the time has come for other experts to join the fight. Since slow onset antibiotic resistance forms the foundation and trigger for an inevitable rapid onset epidemic or pandemic, the use of the emergency risk management process provides a novel way of thinking about responding to the risk now. It provides a useful foundation to engage with the public and others on preparing for and managing the disaster risk of antibiotic resistant bacterial infections generally, and bacterial epidemics and pandemics specifically. The public is, in a sense, already primed to the value of risk management. By analogy, if hundreds or thousands of people were dying each year in bushfires, floods, storms or tropical cyclones, this would be considered a very serious ‘disaster risk management problem’. Bacteria are biological risks to life and as such, they fall within the context of risk management. Given that many thousands of people are dying each year due to the acquisition of antibiotic resistant bacterial infections, the implications for the field of disaster risk management become obvious. This is without consideration of the occurrence of antibiotic resistant bacterial epidemics and pandemics. As such the potential for this approach to be understood by the public is high.
We propose that the issue of antibiotic resistance be rebranded as a disaster risk management problem and that the emergency risk management process be adopted in order to provide new ideas and innovations to address the problem. The emergency risk management process (Figure 1) is described in AS/NZS ISO 31000:2009. The process should be integral to management and decision-making, integrated into practices and culture, and tailored to communities and their risk profiles. In an emergency management context, risk management is a process that involves dealing with risks arising from emergency events (such as the occurrence of bacterial epidemics and pandemics). It is a systematic method for identifying, analysing, evaluating, and treating emergency risks and takes an iterative approach with well-defined activities, leading to implementation of effective risk-treatment strategies. In the case of antibiotic resistance, mitigation measures for sudden onset antibiotic resistant epidemics and pandemics identified by the framework complements measures already implemented by medical health authorities for slow onset antibiotic resistance infections generally.
Figure 1: The emergency risk management process used by governments worldwide.
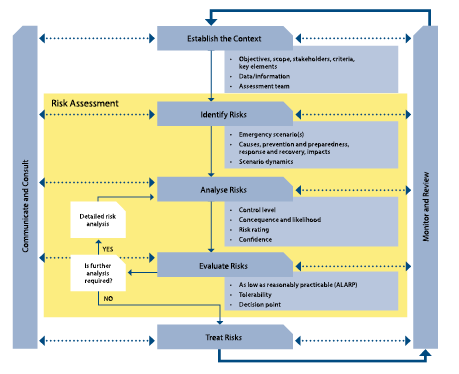
Image taken from the Australian Government Report in National Emergency Risk Assessment Guidelines (Government 2010 p. 13).
The process comprises five elements:
- establishing the context
- identifying the risks
- analysing the risks
- evaluating the risks, and
- treating the risks.
These elements are supported by enabling activities of communicating and consulting, and monitoring and reviewing, which apply to each of the major elements of the process. Risk assessment (the yellow part of Figure 1) also comprises the identification, analysis and evaluation of risk elements of the emergency risk management process. This is important to understand, together with the idea of ‘communication and consultation’ with stakeholders, because high community perceptions and awareness of risk to life is one of the central tenants of effective disaster risk reduction (Bird 2009, Hoppner et al. 2012).
In relation to the emergence of antibiotic resistance and its effect on the risk profile of epidemic and pandemic infections, poor community risk perception is thought to lead to inappropriate behaviours such as patients demanding antibiotics for viral infections or patients failing to complete a course of antibiotics. Given the significance afforded the problem by global organisations like the CDC and the WHO, it is curious that the issue of antibiotic resistance seems so little understood, or of such low concern, to the public generally. This continues despite Option 3 (Antibiotic stewardship) by the WHO (and repeated by others) of engaging with the community through education programs to raise awareness of the issue.
Poor community risk perception is well known in the wider disaster risk reduction literature for influencing appropriate and inappropriate risk behaviour. Significantly, we do not have a comprehensive understanding of how communities perceive antibiotics, their use, and the problem of antibiotic resistance generally. A thorough understanding of these factors is necessary to guide education programs and strategies that reduce risk and increase community resilience. Consequently, we are missing a significant element of the data required to assess the risk as detailed in the emergency risk management process. Addressing the problem of why communities have poor risk perception is easily investigated and one of the simplest challenges to address. Risk managers have a critical and useful role to play. Risk managers can help as they are experienced in investigating why communities behave the way they do, hold the views and perceptions of risks they do, and can communicate complex risk information to the public.
Where to from here
Modification of community behaviour is probably the cheapest and most effective way of dealing with the issue of antibiotic resistance and managing the associated risk. An understanding of the barriers that are preventing appropriate decision-making in the request and prescription of antibiotics is necessary for designing targeted and effective education campaigns to the community and health practitioners. Consequently, in order to address this issue and shift the public discourse to one of increased community engagement and awareness, a rebranding of the issue away from one of ‘general health and medicine’ to one of ‘disaster risk reduction’ would be helpful and represents a novel approach not yet considered. It is our opinion that the emergence of antibiotic resistance is in fact, a risk that ought to be framed as a disaster risk management problem. Consequently, we make an urgent and novel call for the emergency management community and socio-behavioural experts in risk perception to recognise the threat that the emergence of antibiotic resistant bacterial infections represents generally, and antibiotic resistant bacterial epidemics and pandemics specifically. It is our profound view that such an approach would deliver multiple benefits including:
- increased community understanding and awareness of the profound risk to health and life of the emergence of antibiotic resistance generally and the implications for the occurrence of antibiotic resistant bacterial epidemic and pandemic infections specifically
- improved individual and community behaviour (risk management/adaptation) in relation to the risk leading to a reduction in the selection pressure driving the emergence of antibiotic resistance
- enhanced skills and capacity of the health service professionals to treat and support communities facing antibiotic resistant infections, especially epidemics and pandemics
- improved emergency management capacity to anticipate and cope with the risk of antibiotic resistant epidemics and pandemics as well as the emergence of resistant infections in the community, and
- reduced death, suffering, loss and burden of disease.
We strongly urge the emergency management community and risk researchers to join the fight before it is too late – before an antibiotic resistant bacterial epidemic or pandemic strikes.
References
Adger N, Hughes T, Folke C, Carpenter S & Rockstrom J 2005, Socio-ecological resilience to coastal disasters. Science 309: pp. 1036-1039.
Australian Government 2010, National Emergency Risk Assessment Guidelines. Department of Police and Emergency Management. At: ww.em.gov.au/Documents/National%20Emergency%20Risk%20Assessment%20Guidelines%20October%202010.PDF.
Baquero F, Martínez J & Cantón R 2008, Antibiotics and antibiotic resistance in water environments. Curr Opin Biotechnol 19: pp. 260-265.
Bird D 2009, The use of questionnaires for acquiring information on public perception of natural hazards and risk mitigation – a review of current knowledge and practice. Natural Hazards and Earth System Sciences 9: pp. 1307-1325.
Centers for Disease Control and Prevention 2013, Antibiotic resistance threats in the United States 2013.
Francis N, Gillespie D, Nuttall J, Hood K, Little P, Verheij T, Coenen S, Cals J, Goossens H, Butler C & Group GP 2012, Antibiotics for acute cough: an international observational study of patient adherence in primary care. Br J Gen Prac 599: e429-437.
Gilbert L, Iredell J & Merlino J 2014, Healthcare infection prevention and control really is everyone’s business. Microbiology Australia 35: pp. 3-4.
Gottlieb T & Nimmo GR 2011, Antibiotic resistance is an emerging threat to public health: an urgent call to action at the Antimicrobial Resistance Summit 2011. Med J Aust 194: pp. 281-283.
Hoppner C, Whittle R, Brundi M & Buchecker M 2012, Linking social capacities and risk communication in Europe: a gap between theory and practice. Nat Hazards 64: 1753-1778.
Kemper N 2008, Veterinary antibiotics in the aquatic and terrestrial environment. Ecol Indic 8: pp.1-13.
National Prescribing Service Medicine Wise (NPSMW) 2013, Antibiotic resistance is everyone’s problem - be part of the solution. At: www.nps.org.au/media-centre/media-releases/repository/Antibiotic-resistance-is-everyones-problem-be-part-of-the-solution [14 April 2014].
Power E 2006, Impact of antibiotic restrictions: the pharmaceutical perspective. Clin Microb Infect 12: pp. 25-34.
Prasad S & Smith P 2013, Meeting the threat of antibiotic resistance: building a new frontline defence. Australian Government, Office of the Chief Scientist.
Walsh F 2013, Antibiotics resistance ‘as big a risk as terrorism’ - medical chief. BBC News, United Kingdom.
World Health Organization (WHO) 2012, Overcoming antibiotic resistance. World Health Organization Report on Infectious Diseases.
Wright GD 2010, Antibiotic resistance in the environment: a link to the clinic? Curr Opin Microbiol 13: pp. 589-594.
Acknowledgements
The authors thank the Australian Research Council for funding that supported work underpinning this paper via grant number DP130100877.
About the authors
Associate Professor Dale Dominey-Howes is Director of the Asia-Pacific Natural Hazards Research Group, School of Geosciences, University of Sydney. His research expertise is in the field of natural hazards, disaster risk reduction and the socio-cultural dimensions of disasters.
Dr Carolyn Michael is an honorary associate in molecular microbiology at the University of Technology, Sydney. She has extensive experience in clinical pathology as well as infectious disease epidemiology and control.
Dr Maurizio Labbate is Senior Lecturer in Microbiology in the Department of Medical and Molecular Bioscience and the ithree Institute, University of Technology, Sydney. His expertise is in antibiotic resistance, infection and microbial ecology.


